Batteries are fine, but what happens when
you can’t charge them? That’s precisely the problem that faces expeditions to
remote places or just those for whom a power socket isn’t readily available.
The solution is a fuel cell, which, as the
name suggests, converts chemical energy into electricity, allowing
battery-based devices to be recharged or run directly from the fuel cell.
The conventional fuels for these devices
are hydrogen, though other flammable gases and liquids like methanol have also
been used successfully. Their technological origins go back to the Victorian
era, but their value was demonstrated by Nasa, which has used fuel cells on its
spacecraft from its earliest manned missions.

The
solution is a fuel cell, which, as the name suggests, converts chemical energy
into electricity, allowing battery-based devices to be recharged or run
directly from the fuel cell.
So could a fuel cell extend your computing
experience? Yes, and in fact there are a few products close to availability. An
MIT spin-off, Lilliputian Systems of Wilmington Massachusetts, has designed a
tiny USB portable charger that uses cartridges containing lighter fluid to
deliver multiple charges to any compatible device.
The prototype device is about the size of a
thick phone, and can offer between ten and 14 full recharges to a typical
iPhone. The price of the device is expected to be less than $200, and each fuel
cartridge just a few dollars.
The fuel cell has impressed those that have
used it, and Lilliputian Systems has not only been able to crack a deal with
BrookStone to distribute its first product, but it also successfully attained
$60m in equity finance from its investors. Brookstone and Lilliputian will make
a formal product announcement in the coming months, so hopefully ts device will
be another option to extend battery life in 2013.
Nanotubes To The Rescue
Carbon nanotube (that odd organisation of
carbon atoms into very useful structures) have many uses, it’s been discovered.
Graphene, as it’s now being referred to, has some especially interesting
electrical properties, some of which might be incredibly useful for battery
technology.
The one that scientists at MIT first
alighted to was to do with the huge surface area that graphene offers, which is
much greater than the graphite that’s traditionally been used. The first
prototype battery demonstrated in 2010 increased the charge by about 30% more
in the same volume. That’s a modest increase but one that is certainly worth
having. The graphene battery also showed some other unique properties to do
with how rapidly charge could be stored and subsequently released.

The
Tesla Roadster, an super-car that’s powered by batteries. It can travel up to
245 miles on a charge and accelerate to 60mph in just 3.7 seconds, but charging
it from a household electrical outlet could take 60 hours!
That allows the batteries to output
increased power, which could certainly be of use in automotive applications,
but it also allows the batteries to charge must faster too.
For problems that need fast charge and
release, the solution has been to use electrochemical capacitors, but these new
graphene batteries provide a whole new layer between conventional lithium-ion
tech and those more extreme devices.
That begs the question: why aren’t we using
this now? Unfortunately, developing commercial solutions working with tubes
that are just one 50,000th of the thickness of a human hair isn’t a priority
for Yang Shao-Horn, an associate professor of materials science and mechanical
engineering. She’s more interested in understanding the chemistry that their
prototypes use, which has yet to be fully understood.
While this work is interesting in the wider
context of battery technology, it probably won’t be what powers a future phone
or PC. Nevertheless, it might demonstrate a path to making recharging less of a
chore and something that could reduce the impact of the limited carrying
capacity we have now.
However, this isn’t the only way that
nanotubes can help our power needs.
Fatter And Faster
It’s worth considering that part of the
battery life issue is the time it takes to charge, because if it didn’t take so
long, then we’d do it more regularly without much concern.
The most common type of battery used in
computing devices is the lithium-ion variety, which has ousted other chemistry
in the past ten years. The technology of this material has some unusual
properties, some that slow down how rapidly it can be charged. What you might
be unaware of when you’re charging your phone is that the battery grows, as a
charged cell occupies more volume than a depleted one.
Also, the battery is charged from the
outside in, so if you make the battery fatter for greater capacity, it takes
even longer to charge.
You can break the cell into small pieces,
but this only helps a little. A new approach by Korean scientists, working at
the Ulsan National Institute of Science and Technology (UNIST), appears to have
solved this problem.
Their solution is amazingly simple: they
take the cathode material (lithium manganese oxide in this case) and soak it in
a solution containing graphite. That creates fibrous conductive pathways
throughout the cathode, allowing power to penetrate the battery much more
easily. It’s then packaged with the graphite anode component as in a normal
battery and you have a device that is identical in performance, other than it
can charge between 30 and 120 times faster.
The catch? The battery is marginally
bigger, which means that it might not be ideal for phones, but it could be used
in laptops, and it’s perfect for traditionally long-charging technology, like
electric vehicles.
More Anode Options
Using graphite as the anode in batteries
goes back to the very earliest battery designs, because it’s cheap and
plentiful, and it works well enough.
Yet much of the research into new battery
concepts has focused on this part of the battery as being the key to superior
performance.
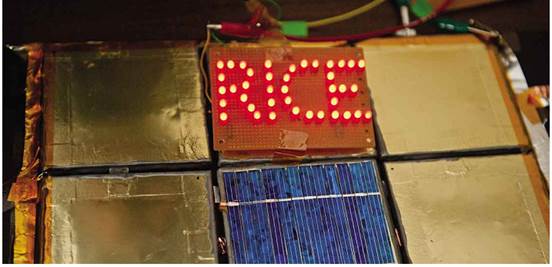
Rice
University has developed a means to spray batteries onto any surface, allowing
them to become part of construction materials
3M, for example, has spent many millions of
dollars and 15 years exploring using silicon as the anode, another cheap and
plentiful material. Why? Well, graphite doesn’t actually store much charge for
its volume, giving a low charge density. The work that 3M has now completed
using silicon boosts the energy density by around 20%, but another 20% extra
power can be had by using new high-energy cathode technology that 3M has also
developed. A total of 40% extra power for the same volume is a significant
improvement and one that most mobile phone and computer users would be keen to
see.
The problem for 3M is that if it doesn’t
get this technology to market soon, it could be overtaken by other developments
in both anode and cathode chemistry.
One of these candidates has been developed
by research company CalBattery, which also uses a silicon anode, but its design
mixes that silicon with graphene.
Dubbed ‘GEN3’ by CalBattery, the anode
substrate it’s developed is a silicon-graphene composite that solves the
problem that many battery chemists have encountered when experimenting with
silicon. Namely, that silicon absorbs lithium better than any other anode
material, but the charge/discharge cycles causes the combination to chemically
alter, providing a short life-span. CalBattery claims to have solved that
problem and in the process delivered triple the power density for the same
volume and mass.
With CalBattery aiming to get products to
market in the next two years, this could be the battery revolution that could
see electric cars become much more practical and phones that work much longer
than a day between recharges.
These are just two of the companies
involved in this line of research, but numerous other companies are investing
heavily in battery research, including General Motors and Envia Systems, who
together are aiming to sell electric vehicles with 200-plus mile ranges in the
next four years. Tesla Motors is also working with PolyPlus, and their
lithium-air and lithium-water battery technology aiming to get 500 miles of
charge in a car. With that level of power density, surely making a phone or
laptop work for longer isn’t just a pipe-dream?